Advisors to the Food and Drug Administration have voted unanimously in support of making a naloxone product available without requiring a prescription, as the pharmaceutical company manufacturing that product doubled down on needle stigma.
In a February 15 joint meeting of the FDA’s Nonprescription Drugs Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee, the committee members voted 19-0 that the FDA should approve a 2022 application for over-the-counter (OTC) naloxone from Emergent BioSolutions—the company that makes Narcan. While the FDA is not legally compelled to act according to the vote, advisory committee members wield substantial influence and historically their recommendation has been a strong predictor of the agency’s final decision.
Emergent’s OTC naloxone would be the same product as its prescription Narcan, just with updated labeling and packaging. It will likely receive FDA approval at the end of March. At that time, any Narcan that had already been distributed bearing the prescription label would become legally considered OTC as well.
In a meeting that lasted over eight hours, the FDA considered whether Emergent’s OTC naloxone product would be safe and effective for community use without the presence of a health care professional. There was only limited acknowledgement of the fact that drug-user communities have been doing this for years, both with nasal Narcan and intramuscular naloxone.
Emergent has long promoted a false narrative that its nasal Narcan sprays are safer and easier to use than generic injectable naloxone, which is a fraction of the cost. Company representatives emphasized Narcan’s “needle-free and easy to carry presentation” and that it was “imperative to develop a naloxone product that can be used without training,” claiming that generic naloxone was “not optimized for use by laypeople.”
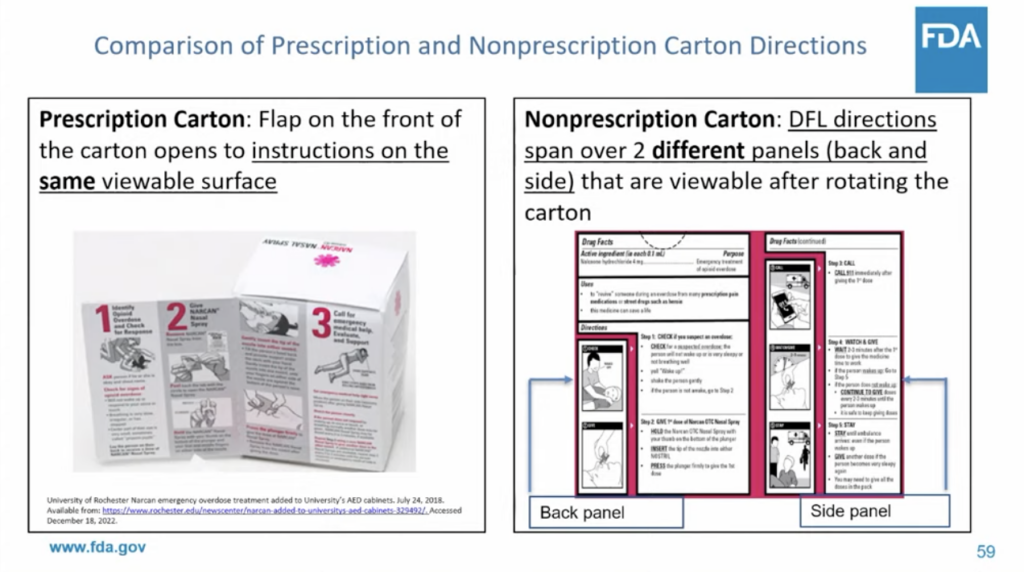
The focus on updated packaging with clear, easily understood instructions is a good thing. In either the intranasal or intramuscular formulation, naloxone is fairly simple to use and should never have had the additional barrier of training requirements; you aren’t required to go through a similar training session when you pick up your other prescription medications.
But Emergent, which made millions off the 2021 affordable naloxone shortage, is leaning heavily into the narrative that intramuscular naloxone—comparable to an EpiPen—is not suitable for use by laypeople. The only appropriate products to approve as OTC, the company claims, are the nasal sprays.
Its FDA application goes as far as citing the potential for needle-stick injuries from generic naloxone, despite what Maya Doe-Simkins, codirector of affordable naloxone distributor Remedy Alliance, described to Filter as an “absolute lack of evidence.”
Timely reminder that the distribution of naloxone outside of health care systems was innovated by drug users and harm reduction programs 27 years ago. pic.twitter.com/m3xgKu3ySE
— Remedy Alliance/For the People (@RemedyAlliance) February 15, 2023
Generic injectable naloxone has a number of benefits over nasal Narcan, including the ability to titrate a dose and avoid precipitated withdrawal (PW). Even Emergent’s application advised that if PW was a concern, it was preferable to use an “alternative, naloxone-containing product that can be titrated.”
Speakers in the joint meeting referred to PW as an inevitable risk of reversals, emphasizing that it’s unpleasant but only life-threatening for infants—despite the fact that PW is known to be life-threatening for adults in carceral settings. The joint meeting focused disproportionately on naloxone access for youth and middle-income consumers.
Another critical benefit of injectable naloxone is cost. Emergent’s branded Narcan has cornered the market despite being significantly more expensive than the generic formulation.
“If we actually, actually want to increase access, to have it where it needs to be and move forward with naloxone saturation, the regulatory issues and the cost cannot be separated,” Doe-Simkins told Filter. “This deals with the regulatory [issue], but does not deal with the cost.”
Update, February 15: This article has been edited to include the outcome of the advisory committee vote.
Top photograph via Oregon Heath Authority. Inset image via Food and Drug Administration.




Show Comments