On August 26, the Food and Drug Administration (FDA) denied the first marketing applications for about 55,000 flavored e-cigarette products from three small companies.
The applicants “lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by well-documented, alarming levels of youth use of such products,” according to an FDA press release. (The evidence does not actually support this stance.)
Earlier in August, the FDA refused to file some 4.5 million product applications from JD Nova, citing that the company had failed to include an adequate environmental assessment for those products. It was largely a bureaucratic technicality to allow the agency to dismiss those applications without fully considering any corresponding scientific or behavioral data that may have been included.
This new rejection amounts to the first outright denial of vaping products through the FDA’s premarket tobacco product application (PMTA) process, which requires manufacturers to prove through a substantive scientific review that their products will be “appropriate for the protection of public health.” The FDA determined that wasn’t the case for the products of three applicants: Great American Vapes, Vapor Salon and (again) JD Nova.
Tobacco harm reduction (THR) advocates were alarmed by the decision, believing the agency was indicating that it would not authorize any bottled e-liquid in flavors except for tobacco. Still, the development—though admittedly not a source of optimism for THR proponents—does not immediately spell disaster.
It remains unclear what the FDA will do by its September 9, 2021, deadline, when it is supposed to decide which vapor products can remain legally on the market. It is also unclear which flavors from which companies—if any—the agency might end up authorizing.
More and more manufacturers are looking at synthetic nicotine as a means of skirting the FDA regulations.
But there’s general concern among vaping manufacturers, consumers and advocates that the FDA will only authorize the companies with the biggest market share, consequently putting a majority of the mom-and-pop vape shops and smaller- and medium-sized producers out of business.
Or those producers will devise new and unique ways to potentially escape from the FDA’s regulatory crosshairs. Like Vapor Salon, the small manufacturer in Fort Worth, Texas, that just had its products denied by the FDA.
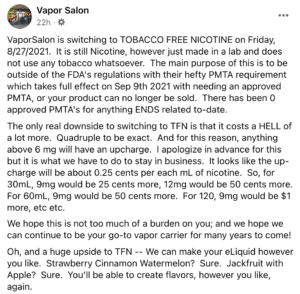
In a public Facebook post, on the same day the company declined to comment for a Washington Post article, Vapor Salon wrote that it would be switching to synthetic nicotine by Friday, August 27—less than 24 hours after the FDA ordered the company to remove its products from the market. (Vapor Salon did not respond to Filter’s request for comment.)
“VaporSalon is switching to TOBACCO FREE NICOTINE on Friday, 8/27/2021,” the post reads. “The main purpose of this is to be outside of the FDA’s regulations with their hefty PMTA requirement which takes full effect on Sept 9th 2021 with needing an approved PMTA, or your product can no longer be sold. There has been 0 approved PMTA’s for anything ENDS related to-date.”

As Filter previously reported, more and more manufacturers have begun looking at the possibility of synthetic nicotine—that is, nicotine made in a lab and not derived from tobacco—as a means of potentially skirting around FDA regulations.
The FDA defines a “tobacco product” as anything “made or derived from tobacco that is intended for human consumption, including any component, part or accessory of a tobacco product.” On the closest and most technical readings, the phrasing would preclude the agency from treating synthetic nicotine as it does other nicotine products, which are almost always derived from tobacco.
Still, synthetic nicotine is expensive—Vapor Salon indicated that many of their rejiggered products will now have “an upcharge”—and it’s unclear how long the FDA will standby if another Wild West-scenario develops, as the agency issues more PMTA denials and additional manufacturers try to inevitably transition to synthetic nicotine.
Eric Lindblom, a senior scholar at Georgetown’s O’Neill Institute for National and Global Health Law and a former director of the FDA’s Center for Tobacco Products Office of Policy, explained recently to Filter that the FDA typically waits to act until a “crisis” emerges or Congress orders the agency to do something. (With vaping, for example, it was the spiraling outcry around teen use.)
“We are unlikely to see many vape businesses close in the immediate aftermath of marketing denial orders.”
Lindblom broke down two potential FDA reactions: The first, which he views as unlikely, is that the agency could assert jurisdiction over synthetic nicotine as a tobacco product and argue that, when the legislation was written, nobody had the foresight to think about synthetic nicotine. The second is that the agency could claim synthetic nicotine doesn’t have the special privileges of a tobacco product—and therefore must be regulated like any other drug.
Nonetheless, it appears regulators have ignored the opinions of manufacturers and THR advocates, who have long said gray and black markets would develop as the federal government instituted more prohibition-like measures.
“Despite the FDA’s best efforts, we are unlikely to see many vape businesses close in the immediate aftermath of marketing denial orders being issued,” Greg Conley, the president of the American Vaping Association, told Filter.
“Synthetic nicotine products still must abide by nationwide age restrictions, but the Center for Tobacco Products lacks the ability to regulate them as ‘tobacco products,’” he continued. “Unless and until the FDA authorizes a sufficient number of flavored products to keep current ex-smokers off of cigarettes, we will support efforts by small businesses to keep offering their products to adult customers.”
Photograph via Shutterstock
The American Vaping Association has provided donations to The Influence Foundation, which operates Filter.





Show Comments