On January 26, the Food and Drug Administration (FDA) authorized three heated tobacco products manufactured by Philip Morris International. The agency issued marketing granted orders (MGOs) for three tobacco-flavored heat sticks—Marlboro Sienna, Marlboro Bronze and Marlboro Amber, all of which can be used with PMI’s IQOS device.
In 2019, the FDA had authorized IQOS and handful of other Marlboro HeatSticks through its premarket tobacco product application process (PMTA), in which PMI had to demonstrate that these products would be “appropriate for the protection of public health”—meaning, in short, that they’d be more likely to help adults who smoke switch to the safer alternative than to introduce a new generation to nicotine. The agency also granted the company the ability to make some modified risk claims in 2020.
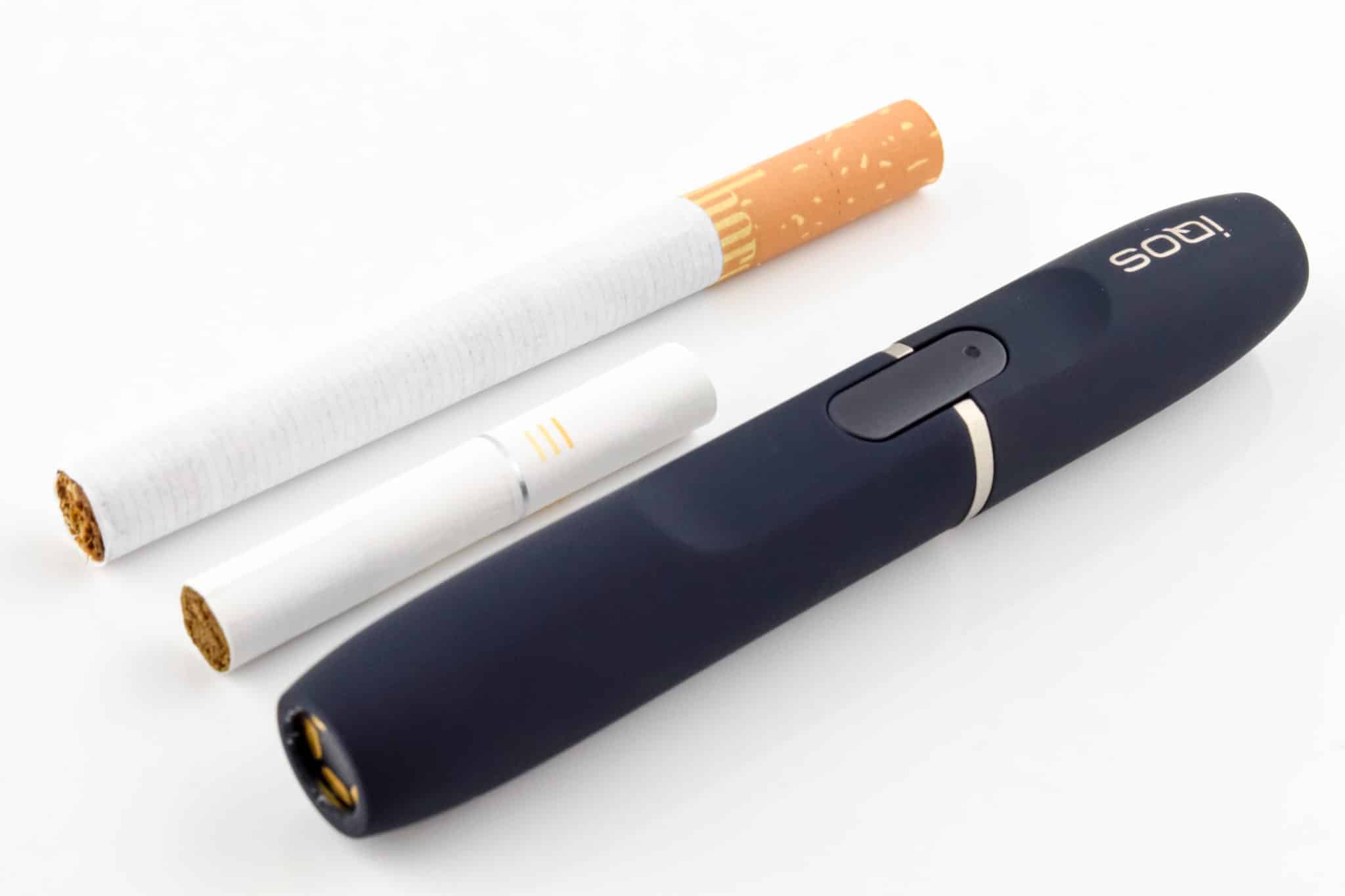
Heated tobacco products (HTPs) heat tobacco sticks without burning them, to produce vapor that is inhaled. Research has suggested that the vapor contains far fewer harmful compounds than cigarette smoke, but probably more than are present in the vapor from e-cigarettes.
It will add to the small availability of FDA-authorized safer nicotine alternatives, but is also a reminder that the PMTA process favors well-financed manufacturers, mainly with ties to the tobacco industry.
HTPs have already taken off in other parts of the world, showing eye-catching potential as a harm reduction option. Most notably, Japan has seen an almost 43 percent decline in cigarette sales over the past decade, right after HTPs were introduced and soared in popularity. And that’s despite the fact that the Japanese government has been, for the most part, rather hostile toward tobacco harm reduction (THR); nicotine vapes, for instance, are effectively outlawed. Critics of mainstream tobacco control—which usually favors prohibition-focused measures—have pointed to Japan as a potential, if limited, model. South Korea has already followed suit, and though Taiwan has just banned even the use of vapes, it has left HTPs alone.
Although the additional IQOS MGOs will add to the small availability of FDA-authorized safer nicotine alternatives in the United States, they are also a reminder that the PMTA process favors well-financed manufacturers, mainly with ties to the tobacco industry.
Still, no flavored vaping products made by anyone have received MGOs. Just days before the IQOS announcement, the FDA rejected two menthol e-cigarettes from Vuse, the R.J. Reynolds-owned vapor company. It left THR advocates and industry observers wondering—again—if the agency will ever authorize any flavored e-cigarettes.
At the moment, IQOS is unavailable in the United States: Altria had begun limited sales of the device in 2019, but a patent dispute with R.J. Reynolds halted any sustained rollout. Then, last October, PMI paid $2.7 billion to regain the US commercialization rights for IQOS from Altria and announced plans to start manufacturing the product in the country.
A new rollout is now set for mid-2024, as the tobacco giant continues to eye the market for safer nicotine alternatives, especially in the US. In November, PMI also took over a majority of Swedish Match—the producer of Zyn, the popular oral nicotine pouch—for $16 billion, inching ever closer toward sole ownership.
Meanwhile, smaller and medium-sized vapor manufacturers—most of which have already had their PMTAs denied—continue to have to fight in court for, perhaps, any hope of an MGO.
Photograph by Vaping360 via Flickr/Creative Commons 2.0
The Influence Foundation, which operates Filter, has received grants from both PMI and Reynolds American, Inc. Filter’s Editorial Independence Policy applies.




Show Comments