Naloxone can reverse an opioid overdose within minutes. Prompt access would prevent many of the 130 opioid-related deaths that occur in the United States every day. A recent CDC analysis of 11,884 opioid-involved overdose deaths across 11 states found that bystanders were present in about 40 percent of cases, but naloxone “was rarely administered by a layperson.” Naloxone is also cheap to make.
So why is it taking so long—and costing so many thousands of lives—to make naloxone accessible to everyone as an over-the-counter (rather than prescribed) medication?
On December 17 and 18 the FDA held a public advisory committee meeting that was intended to “solicit input and advice on strategies on increasing availability of naloxone to reduce opioid overdose deaths,” according to FDA Commissioner Scott Gottlieb.
Yet the only question up for a vote at the end of the two-day meeting was whether the FDA should recommend that naloxone be co-prescribed for “all or some” patients who are prescribed opioids. The committee members voted 12-11 in favor of this recommendation. At best, this step is the bare minimum of what we should be doing. Given that most people who become addicted to opioids didn’t start with a doctor’s prescription, it’s completely inadequate.
Then on January 17, FDA Commissioner Scott Gottlieb announced what the FDA called “unprecedented new efforts to support development of over-the-counter naloxone to help reduce opioid overdose deaths.” This means that the agency has conducted one part of the time-consuming process that a pharmaceutical company would have to go through to submit an OTC naloxone product for approval. The FDA has developed a model Drug Facts Label (DFL) with “easy-to-understand pictograms on how to use the drug” (below) and conducted testing to ensure that people would be able to understand the instructions.
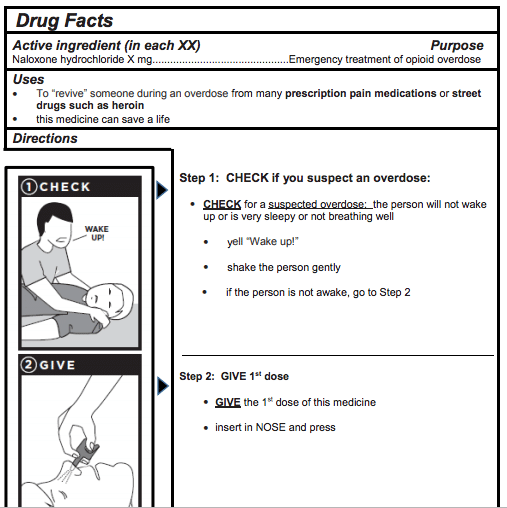
[An excerpt from the FDA’s new model DFL for OTC naloxone nasal spray; there’s also one for use with an auto-injector.]
The FDA’s naloxone DFL has been a long time coming; it was first announced in August 2016. So what now?
“FDA’s development work here is extraordinary, and reflects their longstanding desire to see a pharmaceutical company take naloxone OTC,” says Amy Arthur, director of public and government relations at Harm Reduction Therapeutics, a non-profit trying to develop an over-the-counter naloxone product. “The development of a DFL and testing of its comprehension is of course usually the responsibility of the sponsor, and considerable time and effort usually goes into meeting the FDA’s standards for these critically important communication tools.”
Arthur says that Harm Reduction Therapeutics will be using the FDA’s naloxone DFL in their own OTC development program.
“Responsibility now falls squarely on the shoulders of industry,” she says.
Naloxone was first patented in 1961. It was approved in injectable form by the FDA in 1971, and has been off-patent since 1985. A decade ago, a lifesaving dose of naloxone cost $1. Today, two naloxone products currently have FDA approval for use outside medical facilities by people without medical training. A dose of naloxone in the nasal spray, which was approved in 2015, costs $150. A naloxone auto-injector, approved in 2016, costs $4,500.
As Michael Hufford, PhD, CEO of Harm Reduction Therapeutics, and Donald S. Burke, dean of the Graduate School of Public Health at the University of Pittsburgh, wrote recently in an op-ed for Stat: Naloxone’s “high price and restricted availability—despite its low production costs and excellent safety and effectiveness records—betray our collective ambivalence about the millions of Americans with opioid use disorder.”
 [Timeline via Michael Hufford’s presentation to the FDA]
[Timeline via Michael Hufford’s presentation to the FDA]
Pharma’s Self-Interest
Significantly, the companies that manufacture these expensive naloxone products were represented at the December FDA meeting.
In addition to presentations from FDA employees, and researchers and activists from places like the CDC, Boston University School of Medicine, the VA, the San Fransisco Department of Public Health, the Harm Reduction Coalition and the Drug Policy Alliance, the meeting featured presentations from three Pharma representatives.
These included Robert G. Kramer, president and COO of Adapt Pharma/Emergent Biosolutions, which makes Narcan® Nasal Spray; and Omar Khalil, general manager of Neurology and Addiction for Kaléo, which makes the Evzio® auto-injector.
Dean Mariano, senior director of clinical development/medical affairs for Insys Therapeutics, also presented. Insys is developing a naloxone nasal spray and it has announced plans to submit a New Drug Application (NDA) in 2019.
The three companies released a 23-page joint briefing note for the meeting. Its only mention of “over the counter” was a small section on one slide called “OTC Challenges.” The slide identifies a “challenge” of OTC as: “Risk higher financial barrier for many without improvement in access or awareness.”
That’s pretty rich, considering that they would be the ones setting the price. When meeting attendees asked industry reps “why they oppose OTC access at this time,” they again claimed that they were concerned about cost to consumers—since insurance wouldn’t cover OTC drugs. Yet proponents of OTC naloxone say that going over-the-counter could make it more affordable. Flonase, a nasal spray for allergies, dropped from up to $100 to less than $20 once it became available on store shelves, Mark Kantor, a pharmacist who signed an Oregon petition to make naloxone available OTC, has noted.
The Harm Reduction Coalition’s recommendations at the FDA meeting included “Approve an OTC form of naloxone that will be available to programs doing community-based distribution at low cost (<$1):
.@harmreduction‘s recommendations at today’s FDA adcomm pic.twitter.com/iwvLQFqkYH
— SAC Tracker (@FDAadcomm) December 18, 2018
A Human Rights Watch representative also supported OTC naloxone, calling it “necessary and overdue.” And other experts say that the actual cost of a naloxone dose should be less than $1. In fact, industry insiders say it costs about a nickel.
As it is now, the companies that make naloxone can charge astronomical prices. Comments from Emergent BioSolutions CEO Daniel J. Abdun-Nabi in August made clear that he sees naloxone in profit terms. Announcing that his company was acquiring Adapt Pharma, he described US high schools and colleges as an untapped market. It’s a “growth opportunity,” he said, according to Bloomberg.
Kaléo, meanwhile, made recent headlines when it lowered the cost of its naloxone product to $178 for two injectors. But that was only after a Senate investigation found that it had hiked the price of its drug Evzio to $4,100 for two injectors, an increase of more than 600 percent between 2014 and 2017.
Harm Reduction Therapeutics Leads Quest for OTC Naloxone
The mission of Harm Reduction Therapeutics is to prevent opioid-related deaths by making low-price naloxone available to everyone. Founded in 2017 in response to the severe price and access limits of existing naloxone products, the non-profit pharma organization brings together experts in drug development, harm reduction, substance dependence, public health policy and over-the-counter switches of prescription pharmaceuticals.
The team behind Harm Reduction Therapeutics has over two decades of history of Rx-to-OTC switch successes, including Plan B and Nicorette. In 2018, they received an unrestricted grant of $3.42 million for the development of an over-the-counter (OTC) naloxone nasal spray from Purdue Pharma—the producer of OxyContin and the most high-profile company widely blamed for the opioid addiction crisis. “This grant is one example of the meaningful steps Purdue is taking to help address opioid abuse in our communities,” said Craig Landau, MD, president and CEO of Purdue Pharma in a press release.
An approach taken by many states has been for a high-ranking state health official to issue a “standing order” for naloxone. This is essentially a prescription for everyone, making it possible to obtain naloxone from a pharmacist without first having to visit a doctor to get a prescription. While appealing in principle, this approach hasn’t worked for three reasons, according to CEO Michael Hufford: Most citizens are unaware of these programs; many people don’t have insurance, while those who do may be afraid to use it for naloxone due to stigma and the risk of punitive repercussions; and the price remains prohibitive.
“We offer a different solution,” Hufford says. “Make naloxone available over the counter, in much greater quantities and at lower prices.”
Proponents say that naloxone meets the criteria needed to be OTC. OTC drug products have these general characteristics:
*Their benefits outweigh their risks
*The potential for misuse and abuse is low
*Consumer can use them for self‐diagnosed conditions
*They can be adequately labeled
*Health practitioners are not needed for the safe and effective use of the product
Harm Reduction Therapeutics anticipates its OTC naloxone product will be developed, approved and distributed in 18–24 months. (Given the nature of this grant, no revenues or royalties will be paid to Purdue.)
In the meantime, there are two ways for the FDA to make naloxone available OTC, Hufford explains. The FDA “can’t dictate by fiat that a certain drug is approved over the counter.” Instead, pharma companies must come forward with an application. A “Citizens petition,” like the one filed in Orgeon, is a second potential route.
The non-profit consumer rights group Public Citizen, which was also present at the FDA meeting, says the federal government should procure generic naloxone kits under Government Patent Use law (28 USC Section 1498). Others have argued the same—or at least that the government should use this statute to “bring [pharmaceutical companies] to the negotiating table.”
“The most recent use of § 1498 in the pharmaceutical context was in 2001,” stated Public Citizen in a letter to the FDA committee, “when then-Secretary of Health and Human Services, Tommy Thompson, publicly considered using the authority to procure generic ciprofloxacin during the post-September 11th anthrax scare. The ciprofloxacin patent holder, Bayer, quickly cut its prices in half.”
The “high price of naloxone treatments is neither inevitable nor insurmountable,” Public Citizen continued. “Instead, the high price reflects a set of policy choices by the federal government to refrain from using its existing authority to increase access to affordable generic treatment. These choices must change.”
The fastest way for naloxone to become available OTC, according to Hufford, is for a company with an existing product (like Adapt Pharma/Emergent Biosolutions or Kaléo) to file to take it OTC.
Filter asked the FDA whether they believe the new DFL will motivate those companies to actually develop OTC naloxone. A representative responded: “We hope that by conducting the consumer testing and developing the model DFL, which takes time, that companies will view this as removing a barrier to development.”
Given the strong past opposition to OTC of the companies currently charging sky-high prices for naloxone, it seems unlikely that they will be swayed by the FDA’s DFL move. But they aren’t the only game in town.
“If they won’t,” says Hufford, “we will.”
Main image via Libreshot




